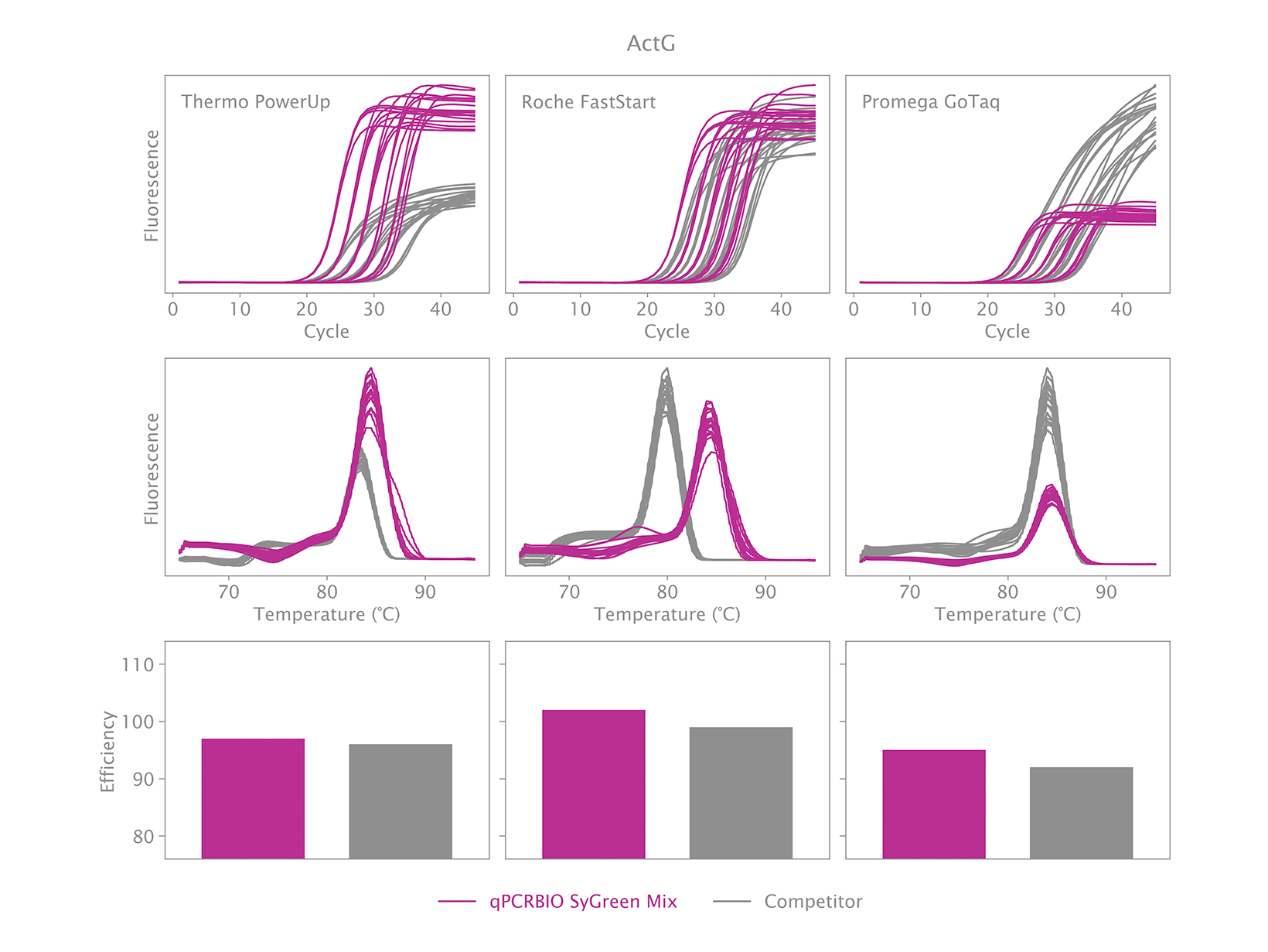
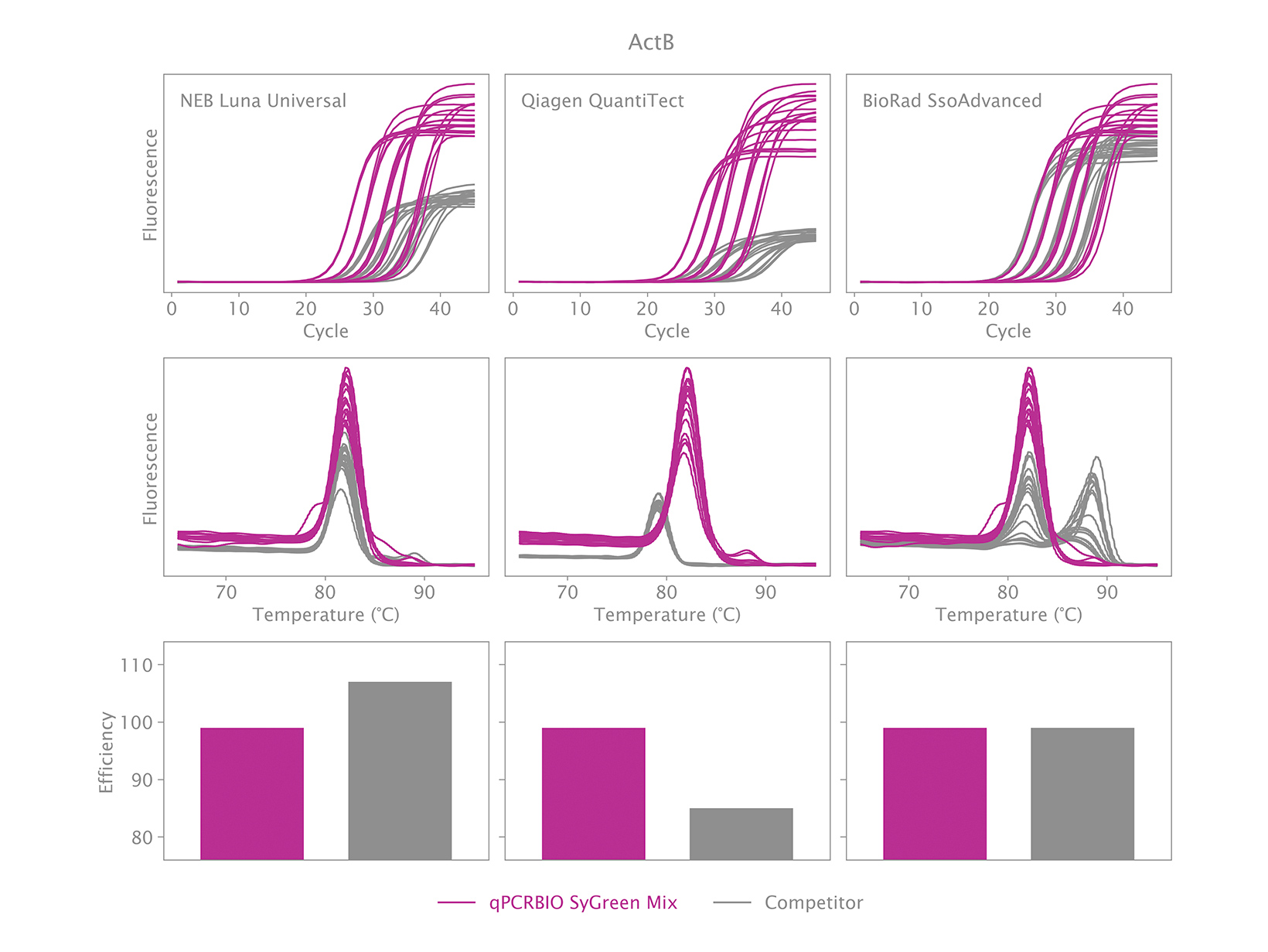
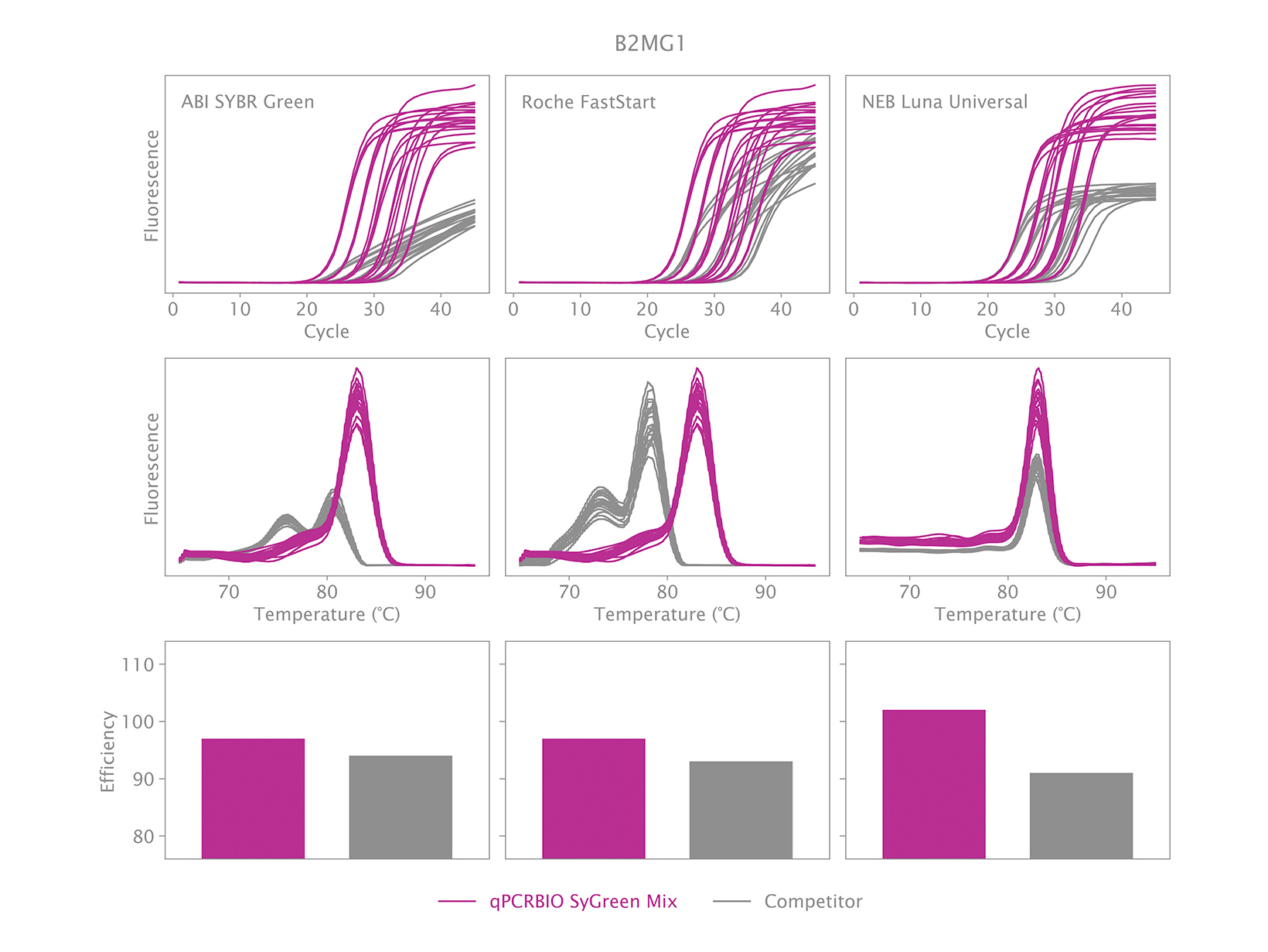
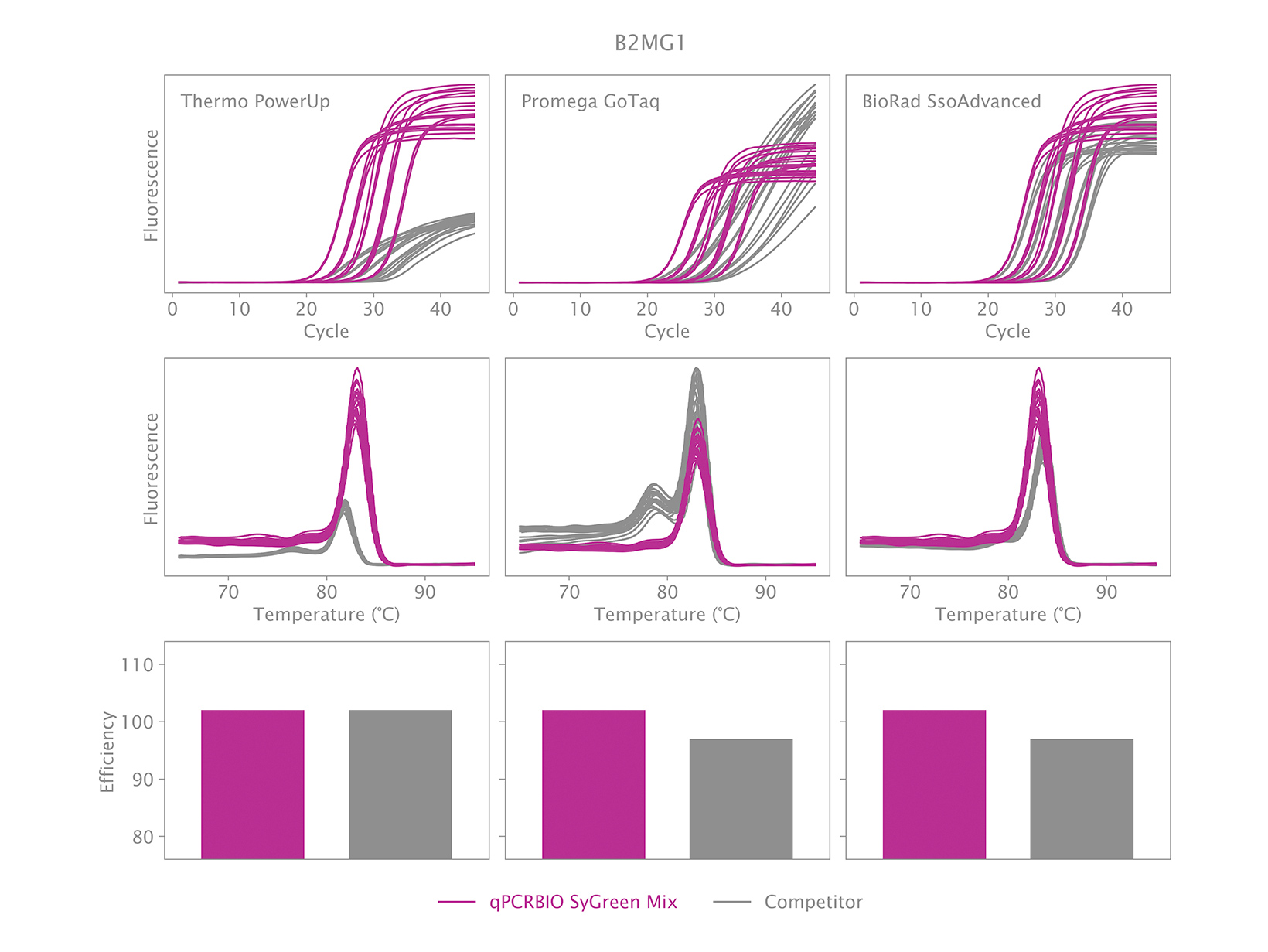
To view your price please login or contact us
qPCRBIO SyGreen Mix
qPCRBIO SyGreen Mix combines a proprietary non-inhibiting intercalating dye with the latest advances in polymerase technology and buffer chemistry to give you fast, highly sensitive and reproducible dye-based real-time qPCR.
qPCRBIO SyGreen Mix can be used to reliably quantify any DNA template including genomic, cDNA and viral sequences using dye-based qPCR, and is able to detect extremely low copy number targets with the highest efficiency.
Features
- Rapid extension rate for early Ct values
- Market-leading sensitivity – increased limit of detection
- Specific amplification from complex templates including GC-rich sequences
- Non-PCR inhibiting intercalating dye
- Also available as an easy-to-see blue mix
- Compatible with all standard and fast cycling real-time instruments
More Information
qPCRBIO SyGreen Mix is designed for fast, highly sensitive and reproducible dye-based real-time qPCR1, with minimal or no optimisation required. The antibody-mediated hot start system prevents the formation of primer dimers and non-specific products leading to greatly improved reaction sensitivity and specificity.
Our 2x mix uses a proprietary DNA intercalating dye, that does not inhibit PCR, unlike other popular fluorescent dyes. The same developments that improve the sensitivity and consistency of qPCRBIO SyGreen Mix in standard cycling conditions also allow for industry-leading performance in fast and ultra fast cycling conditions.
Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
How does qPCRBIO SyGreen work?
These intercalating dyes possess aromatic and planar groups that allow the intercalation of the molecule in the minor groove of double-stranded DNA spanning approximately 3.5-4 nucleotides. The positive groups present stabilise the dyes allowing for the coordination with the negatively charged DNA backbone.
Due to this mechanism of binding to DNA, they are not able to bind to single-strand nucleic acids, unless secondary structures are formed. On the other hand, fluorescence increases up to 1,000-fold upon intercalation with dsDNA.
When a dye intercalates between DNA base pairs, it emits fluorescence proportional to the amount of bound dye (and hence to the amount of DNA in a sample). This allows visualisation of nucleic acids on gels or fluorimeters in real time qPCR instruments. In the latter case, the number of intercalated dye molecules in a sample increases with the progress of a qPCR, leading to a corresponding increase in fluorescence with each cycle. At the onset of the reaction, the free dye emits a basal fluorescence level. As the reaction proceeds the fluorescence intensity increases exponentially, before it plateaus in later cycles, leading to the classic sigmoidal amplification plot.
Benefits and Limitations of Dye-Based qPCR
Benefits:
Versatile Target Detection: By combining a SyGreen or SYBR Green-type dye with primers, researchers can detect any target molecule of interest. This versatility allows for simple and cost-effective design of high-throughput singleplex qPCRs.
Reversible Fluorescent Signal Accumulation: The use of a DNA intercalating dye facilitates reversible accumulation of fluorescent signal. Because strong fluorescence arises only from intercalated dye, denaturation of DNA leads to a reduction in fluorescent signal. This characteristic enables specificity testing through melt curve analysis, which in turn allows for rapid evaluation of qPCR specificity (see more below).
Limitations:
Non-Specific Signal Generation: The intercalating dye can generate non-specific fluorescent signals when it binds to any dsDNA molecule present in the reaction tube. This can occur due to mis-priming, the presence of similar sequences in the sample, the formation of primer dimers, or potential primer hairpin structures. To validate results, additional analyses like melt curve analysis, gel electrophoresis, or Sanger sequencing should be performed.
Single Target Assay: Dye-based qPCR allows only one target to be assayed per reaction. For quantifying multiple targets in a sample, a corresponding number of reactions must be set up.
Melt Curve Analysis
Melt curve analysis serves to identify the melting point of a qPCR product, providing valuable insights into product specificity. This analysis involves an additional thermocycling step at the end of the qPCR cycling program. During this step, the reaction samples are gradually denatured by incrementally increasing the incubation temperature while collecting fluorescence data at each temperature interval (e.g., every 0.1-0.5 °C). Plotting the fluorescence intensity data against temperature generates the melt curve. In a reaction containing a specific product, fluorescence remains steady until the temperature approaches the target’s melting point, at which point it rapidly drops to baseline. The temperature at which fluorescence decreases to 50% of the maximum is considered the melting point or Tm of that molecule. Evaluating reaction specificity can be simplified by plotting the first-order derivative of fluorescence intensity (-dRFU) against temperature change (dT), known as dRFU/dT. For a reaction with a single specific product, this results in a parabolic peak centered around the product’s Tm, known as a melt peak.
For more in depth information on dye-based qPCR or qPCR in general please refer to our technical guide.
Disclaimer:
SYBR Green is a registered trademark of Molecular Probes Inc. and is owned by Thermo Fischer Scientific. PCR Biosystems Ltd. is in no way affiliated with any of these entities.
Applications
- Absolute quantification
- Relative gene expression analysis
- High-throughput qPCR from genomic, cDNA and viral sequences
- Detection of extremely low copy number targets
- Crude sample PCR
Specifications
qPCRBIO SyGreen Mix Lo-ROX
Component
100 Reactions
500 Reactions
2000 Reactions
5000 Reactions
5000 Reactions
2x qPCRBIO SyGreen Mix Lo-ROX
1 x 1mL
5 x 1mL
20 x 1mL
1 x 50mL Bottle
50 x 1mL in Pouch
qPCRBIO SyGreen Mix High-ROX
Component
100 Reactions
500 Reactions
2000 Reactions
5000 Reactions
5000 Reactions
2x qPCRBIO SyGreen Mix Hi-ROX
1 x 1mL
5 x 1mL
20 x 1mL
1 x 50mL Bottle
50 x 1mL in Pouch
qPCRBIO SyGreen Mix with Fluorescein
Component
100 Reactions
500 Reactions
2000 Reactions
2x qPCRBIO SyGreen Mix Fluorescein
1 x 1mL
5 x 1mL
20 x 1mL
qPCRBIO SyGreen Mix Separate-ROX
Component
100 Reactions
500 Reactions
2000 Reactions
5000 Reactions
5000 Reactions
2x qPCRBIO SyGreen No-ROX
1 x 1mL
5 x 1mL
20 x 1mL
1 x 50mL Bottle
50 x 1mL in Pouch
50μM ROX Additive
1 x 200μL
1 x 200μL
4 x 200μL
2 x 520μL
2 x 520μL
qPCRBIO SyGreen Mix Lo-ROX
Component
2x qPCRBIO SyGreen Mix Lo-ROX
100 Reactions
1 x 1mL
500 Reactions
5 x 1mL
2000 Reactions
20 x 1mL
5000 Reactions
1 x 50mL Bottle
5000 Reactions
50 x 1mL in Pouch
qPCRBIO SyGreen Mix High-ROX
Component
2x qPCRBIO SyGreen Mix Hi-ROX
100 Reactions
1 x 1mL
500 Reactions
5 x 1mL
2000 Reactions
20 x 1mL
5000 Reactions
1 x 50mL Bottle
5000 Reactions
50 x 1mL in Pouch
qPCRBIO SyGreen Mix with Fluorescein
Component
2x qPCRBIO SyGreen Mix Fluorescein
100 Reactions
1 x 1mL
500 Reactions
5 x 1mL
2000 Reactions
20 x 1mL
qPCRBIO SyGreen Mix Separate-ROX
Component
2x qPCRBIO SyGreen No-ROX
50μM ROX Additive
100 Reactions
1 x 1mL
1 x 200μL
500 Reactions
5 x 1mL
1 x 200μL
2000 Reactions
20 x 1mL
4 x 200μL
5000 Reactions
1 x 50mL Bottle
2 x 520μL
5000 Reactions
50 x 1mL in Pouch
2 x 520μL
Reaction Volume
Storage
20μL
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Reaction Volume
20μL
Storage
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Instrument Compatibility
This product is compatible with all standard and fast cycling qPCR instruments. Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
Documents
Product Flyers
Product Manuals
Material Safety Data Sheets
Certificate of Analysis Finder
FAQs
Can products generated with qPCRBIO SyGreen Mixes be digested, cloned, and sequenced?
Yes, PCR products generated with qPCRBIO SyGreen Mixes have the same characteristics as PCR products generated with wild-type Taq polymerase. They may be sequenced or digested with restriction endonucleases using standard protocols. Products are 3′-d(A)-tailed and may be used for TA cloning or may be blunt-ended or digested with restriction enzymes prior to cloning. For best results, we recommend purifying the PCR products using any standard PCR clean-up kit.
Can ROX have a negative impact on the reaction?
ROX (6-carboxy-X-rhodamine) is used as a passive reference dye in ROX-dependent real-time PCR instruments to normalize for variations of fluorescence levels that can arise mainly due to optical path variations among wells. Normalisation of the fluorescence intensity (Rn) is done in real-time PCR software by dividing the emission intensity of the specific signal by the emission intensity of ROX.
ROX does not take part in the PCR reaction and its fluorescence levels are not proportional to the quantity of DNA in each well, so the addition of this fluorophore to a mix provides a constant fluorescent signal during amplification.
Different types of real-time PCR instruments requiring a passive reference standard have different optimal concentrations of ROX, mainly due to the different optical configurations of each system (i.e. the different type of excitation source and optics used).
The addition of either too little or too much ROX would result in a very noisy signal impacting on the results of the reaction. Therefore, it is extremely important for the user to:
- Determine the correct ROX concentration to optimise real-time PCR results, and
- Check the ROX settings on the software used to set up the reaction
A useful selection tool for the most commonly used systems can be found here.
Do qPCRBIO SyGreen Mixes work with bisulphate converted gDNA template?
Yes. We have customers who have reported successful use of qPCRBIO SyGreen Mix on bisulphate converted gDNA.
Is the storage of sample DNA in 1x TE (10 mM Tris-HCl / 1 mM EDTA) buffer compatible with subsequent qPCR using qPCRBIO SyGreen mixes?
Yes, this storage buffer is compatible. The EDTA will chelate some of the magnesium in the mix, but not significantly enough to affect the reaction.
qPCRBIO SyGreen Mixes contain the dye SyGreen. Does it belong to the SYBR green family?
SyGreen belongs to the family of asymmetric cyanine dyes, which is the same family that SYBR™ Green belongs to. SyGreen is an improved, new generation dye from this family. Similar to SYBR™ Green, SyGreen intercalates the DNA. Unlike SYBR™ Green, SyGreen supports PCR amplification over a wide range of dye concentrations, that is, the dye does not inhibit the PCR reaction. This plays a role in producing robust and consistent DNA melting curves both with prokaryote and eukaryote templates, as it shows no preference in nucleotide composition.
What are the ROX concentrations in qPCRBIO SyGreen Mixes?
The qPCRBIO SyGreen Mixes that contain passive reference dyes come in different formulations, each with a different concentration of the passive reference dye:
- qPCRBIO SyGreen Mix Lo-ROX (PB20.11) contains 112 nM ROX.
- qPCRBIO SyGreen Mix Hi-ROX (PB20.12) contains 1.12 µM ROX.
- qPCRBIO SyGreen Mix Fluorescein (PB20.13) contains 0.2 mM fluorescein.
- qPCRBIO SyGreen Mix Separate-ROX (PB20.14) 2x mix contains no ROX and include a separate tube of 50 µM ROX additive. This enables you to choose what concentration of ROX you’d like to use.
You can use our qPCR Selection Tool under the Resources drop-down menu to determine which of our mixes are best suited for your qPCR machine.
What is included in qPCRBIO SyGreen Mixes?
qPCRBIO SyGreen Mixes are ready to use qPCR 2x Mastermixes. You only need to add primers, template DNA and PCR grade water during reaction set up.
What is ROX?
ROX is a passive reference dye which means it does not take part in the PCR reaction. It is used to normalise non-PCR related fluctuations in fluorescence.
What is the error rate of the HS Taq DNA Polymerase used in qPCRBIO SyGreen Mixes?
The enzyme has an error rate of approximately 1 error per 2.0 x 10⁵ nucleotides incorporated.
What is the MgCl2 concentration in qPCRBIO SyGreen Mixes?
All qPCRBIO SyGreen Mixes contain MgCl2 at a concentration of 6 mM. This means the final concentration in the reaction is 3 mM.
What should be considered if the normalisation of signal with ROX appears to be lower relative to a competitor’s mix?
Ensure you are using the right concentration of ROX because different instruments require different ROX concentrations. For example, if a qPCRBIO SyGreen Mix Hi-ROX is used with an instrument requiring a Lo-ROX mix, the software will normalise the qPCRBIO signal against the Hi-ROX level. This will significantly reduce the fluorescence level of the qPCRBIO mix relative to competitors’ mixes, if in that case the Lo-ROX mix was chosen for the competitor.
When comparing mixes from different manufacturers, it is better to carry out separate runs or turn the passive reference off before analysing data.
What troubleshooting is recommended if efficiency of amplification is reduced with standard dilutions?
It has been reported that efficiency can decrease with subsequent dilutions for the standard curve. We recommend avoiding this by diluting the standards in 10 mM Tris-HCl pH 8.0, 0.1 mM EDTA, 0.05% Tween-20. EDTA is a chelating agent and it plays a role in preventing DNAse activity1. Tween-20 is a detergent and prevents the DNA from adsorbing to the sides of the tubes2. Most microcentrifuges are made of polypropylene and research has demonstrated that DNA sticks very well to polypropylene3.
Standards should not be frozen after diluting them. Even in the presence of detergent, freezing seems to cause DNA to bind irreversibly to polypropylene. We suggest leaving your standards at 4°C and preparing a fresh batch every few weeks.
1 Barra, G. B. et al. EDTA-mediated inhibition of DNases protects circulating cell-free DNA from ex vivo degradation in blood samples. Clin Biochem 48, 976-981, doi:10.1016/j.clinbiochem.2015.02.014 (2015).
2 Linnarsson, S. Recent advances in DNA sequencing methods – general principles of sample preparation. Exp Cell Res 316, 1339-1343, doi:10.1016/j.yexcr.2010.02.036 (2010).
3 Gaillard, C. & Strauss, F. Avoiding adsorption of DNA to polypropylene tubes and denaturation of short DNA fragments. Technical Tips Online 3, 3 (1998).
What troubleshooting is recommended if the background signal is very high?
A high background level of fluorescence is most likely due to an excess of template in the reaction. This relates to the qPCR instrument picking up the SyGreen dye which binds to the template DNA. Samples usually contain a lot of DNA other than the target gene, so there will be enough present to register fluorescence. We recommend diluting the samples 100x-1000x to overcome this issue.
This should not only reduce the background signal, but also allow an accurate quantification because the dilution should bring the Ct values in the range of those obtained with the standards. Keep in mind that if the Ct is earlier than the first standard, it won’t be considered accurate enough quantification for publication.
Why is there a non-specific product when using the same primers and PCR conditions as a competitor’s product?
It’s most likely because the time for the 1st step (hot start) is too short. Ensure that the hot start phase is done at 95°C for 2 minutes to fully activate the enzyme. The recommended thermal profile is:
- 95°C (120 seconds)
- 40 cycles: 95°C (5-15 seconds) – 60°C (20-30 seconds)
- Melt
If non-specific products are still obtained, we recommend raising the annealing/extension temperature from 60°C to 65°C, depending on the primer set used.
Why is there an earlier Ct when using qPCRBIO SyGreen Mixes compared to a competitor’s product?
An early Ct could indicate one of two things. The first could be the result of our product performing very well relative to the competitor’s product. We have designed our products to be faster than our competitors, and we have found that we are significantly faster than many.
The second could be that caused by non-specific products. This can be verified by running a melt analysis at the end of the qPCR run. If your results show one peak, it is likely the correct product was amplified. You may observe the position of the peak being different with our mix relative to our competitor’s mix but is this normal because the salt concentration and pH affect the melting temperature. If your results show more than one peak, then the reaction is not specific. PCR products can also be run on an agarose gel to verify if there is only one band, and if it is the same size as the amplicon obtained with the competitor’s product.
To address the issue of a non-specific product, the thermal cycling will need to be adjusted.
More Information
qPCRBIO SyGreen Mix is designed for fast, highly sensitive and reproducible dye-based real-time qPCR1, with minimal or no optimisation required. The antibody-mediated hot start system prevents the formation of primer dimers and non-specific products leading to greatly improved reaction sensitivity and specificity.
Our 2x mix uses a proprietary DNA intercalating dye, that does not inhibit PCR, unlike other popular fluorescent dyes. The same developments that improve the sensitivity and consistency of qPCRBIO SyGreen Mix in standard cycling conditions also allow for industry-leading performance in fast and ultra fast cycling conditions.
Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
How does qPCRBIO SyGreen work?
These intercalating dyes possess aromatic and planar groups that allow the intercalation of the molecule in the minor groove of double-stranded DNA spanning approximately 3.5-4 nucleotides. The positive groups present stabilise the dyes allowing for the coordination with the negatively charged DNA backbone.
Due to this mechanism of binding to DNA, they are not able to bind to single-strand nucleic acids, unless secondary structures are formed. On the other hand, fluorescence increases up to 1,000-fold upon intercalation with dsDNA.
When a dye intercalates between DNA base pairs, it emits fluorescence proportional to the amount of bound dye (and hence to the amount of DNA in a sample). This allows visualisation of nucleic acids on gels or fluorimeters in real time qPCR instruments. In the latter case, the number of intercalated dye molecules in a sample increases with the progress of a qPCR, leading to a corresponding increase in fluorescence with each cycle. At the onset of the reaction, the free dye emits a basal fluorescence level. As the reaction proceeds the fluorescence intensity increases exponentially, before it plateaus in later cycles, leading to the classic sigmoidal amplification plot.
Benefits and Limitations of Dye-Based qPCR
Benefits:
Versatile Target Detection: By combining a SyGreen or SYBR Green-type dye with primers, researchers can detect any target molecule of interest. This versatility allows for simple and cost-effective design of high-throughput singleplex qPCRs.
Reversible Fluorescent Signal Accumulation: The use of a DNA intercalating dye facilitates reversible accumulation of fluorescent signal. Because strong fluorescence arises only from intercalated dye, denaturation of DNA leads to a reduction in fluorescent signal. This characteristic enables specificity testing through melt curve analysis, which in turn allows for rapid evaluation of qPCR specificity (see more below).
Limitations:
Non-Specific Signal Generation: The intercalating dye can generate non-specific fluorescent signals when it binds to any dsDNA molecule present in the reaction tube. This can occur due to mis-priming, the presence of similar sequences in the sample, the formation of primer dimers, or potential primer hairpin structures. To validate results, additional analyses like melt curve analysis, gel electrophoresis, or Sanger sequencing should be performed.
Single Target Assay: Dye-based qPCR allows only one target to be assayed per reaction. For quantifying multiple targets in a sample, a corresponding number of reactions must be set up.
Melt Curve Analysis
Melt curve analysis serves to identify the melting point of a qPCR product, providing valuable insights into product specificity. This analysis involves an additional thermocycling step at the end of the qPCR cycling program. During this step, the reaction samples are gradually denatured by incrementally increasing the incubation temperature while collecting fluorescence data at each temperature interval (e.g., every 0.1-0.5 °C). Plotting the fluorescence intensity data against temperature generates the melt curve. In a reaction containing a specific product, fluorescence remains steady until the temperature approaches the target’s melting point, at which point it rapidly drops to baseline. The temperature at which fluorescence decreases to 50% of the maximum is considered the melting point or Tm of that molecule. Evaluating reaction specificity can be simplified by plotting the first-order derivative of fluorescence intensity (-dRFU) against temperature change (dT), known as dRFU/dT. For a reaction with a single specific product, this results in a parabolic peak centered around the product’s Tm, known as a melt peak.
For more in depth information on dye-based qPCR or qPCR in general please refer to our technical guide.
Disclaimer:
SYBR Green is a registered trademark of Molecular Probes Inc. and is owned by Thermo Fischer Scientific. PCR Biosystems Ltd. is in no way affiliated with any of these entities.
Applications
- Absolute quantification
- Relative gene expression analysis
- High-throughput qPCR from genomic, cDNA and viral sequences
- Detection of extremely low copy number targets
- Crude sample PCR
Specifications
qPCRBIO SyGreen Mix Lo-ROX
Component
100 Reactions
500 Reactions
2000 Reactions
5000 Reactions
5000 Reactions
2x qPCRBIO SyGreen Mix Lo-ROX
1 x 1mL
5 x 1mL
20 x 1mL
1 x 50mL Bottle
50 x 1mL in Pouch
qPCRBIO SyGreen Mix High-ROX
Component
100 Reactions
500 Reactions
2000 Reactions
5000 Reactions
5000 Reactions
2x qPCRBIO SyGreen Mix Hi-ROX
1 x 1mL
5 x 1mL
20 x 1mL
1 x 50mL Bottle
50 x 1mL in Pouch
qPCRBIO SyGreen Mix with Fluorescein
Component
100 Reactions
500 Reactions
2000 Reactions
2x qPCRBIO SyGreen Mix Fluorescein
1 x 1mL
5 x 1mL
20 x 1mL
qPCRBIO SyGreen Mix Separate-ROX
Component
100 Reactions
500 Reactions
2000 Reactions
5000 Reactions
5000 Reactions
2x qPCRBIO SyGreen No-ROX
1 x 1mL
5 x 1mL
20 x 1mL
1 x 50mL Bottle
50 x 1mL in Pouch
50μM ROX Additive
1 x 200μL
1 x 200μL
4 x 200μL
2 x 520μL
2 x 520μL
qPCRBIO SyGreen Mix Lo-ROX
Component
2x qPCRBIO SyGreen Mix Lo-ROX
100 Reactions
1 x 1mL
500 Reactions
5 x 1mL
2000 Reactions
20 x 1mL
5000 Reactions
1 x 50mL Bottle
5000 Reactions
50 x 1mL in Pouch
qPCRBIO SyGreen Mix High-ROX
Component
2x qPCRBIO SyGreen Mix Hi-ROX
100 Reactions
1 x 1mL
500 Reactions
5 x 1mL
2000 Reactions
20 x 1mL
5000 Reactions
1 x 50mL Bottle
5000 Reactions
50 x 1mL in Pouch
qPCRBIO SyGreen Mix with Fluorescein
Component
2x qPCRBIO SyGreen Mix Fluorescein
100 Reactions
1 x 1mL
500 Reactions
5 x 1mL
2000 Reactions
20 x 1mL
qPCRBIO SyGreen Mix Separate-ROX
Component
2x qPCRBIO SyGreen No-ROX
50μM ROX Additive
100 Reactions
1 x 1mL
1 x 200μL
500 Reactions
5 x 1mL
1 x 200μL
2000 Reactions
20 x 1mL
4 x 200μL
5000 Reactions
1 x 50mL Bottle
2 x 520μL
5000 Reactions
50 x 1mL in Pouch
2 x 520μL
Reaction Volume
Storage
20μL
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Reaction Volume
20μL
Storage
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Instrument Compatibility
This product is compatible with all standard and fast cycling qPCR instruments. Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
Documents
Product Flyers
Product Manuals
Material Safety Data Sheets
Certificate of Analysis Finder
FAQs
Can products generated with qPCRBIO SyGreen Mixes be digested, cloned, and sequenced?
Yes, PCR products generated with qPCRBIO SyGreen Mixes have the same characteristics as PCR products generated with wild-type Taq polymerase. They may be sequenced or digested with restriction endonucleases using standard protocols. Products are 3′-d(A)-tailed and may be used for TA cloning or may be blunt-ended or digested with restriction enzymes prior to cloning. For best results, we recommend purifying the PCR products using any standard PCR clean-up kit.
Can ROX have a negative impact on the reaction?
ROX (6-carboxy-X-rhodamine) is used as a passive reference dye in ROX-dependent real-time PCR instruments to normalize for variations of fluorescence levels that can arise mainly due to optical path variations among wells. Normalisation of the fluorescence intensity (Rn) is done in real-time PCR software by dividing the emission intensity of the specific signal by the emission intensity of ROX.
ROX does not take part in the PCR reaction and its fluorescence levels are not proportional to the quantity of DNA in each well, so the addition of this fluorophore to a mix provides a constant fluorescent signal during amplification.
Different types of real-time PCR instruments requiring a passive reference standard have different optimal concentrations of ROX, mainly due to the different optical configurations of each system (i.e. the different type of excitation source and optics used).
The addition of either too little or too much ROX would result in a very noisy signal impacting on the results of the reaction. Therefore, it is extremely important for the user to:
- Determine the correct ROX concentration to optimise real-time PCR results, and
- Check the ROX settings on the software used to set up the reaction
A useful selection tool for the most commonly used systems can be found here.
Do qPCRBIO SyGreen Mixes work with bisulphate converted gDNA template?
Yes. We have customers who have reported successful use of qPCRBIO SyGreen Mix on bisulphate converted gDNA.
Is the storage of sample DNA in 1x TE (10 mM Tris-HCl / 1 mM EDTA) buffer compatible with subsequent qPCR using qPCRBIO SyGreen mixes?
Yes, this storage buffer is compatible. The EDTA will chelate some of the magnesium in the mix, but not significantly enough to affect the reaction.
qPCRBIO SyGreen Mixes contain the dye SyGreen. Does it belong to the SYBR green family?
SyGreen belongs to the family of asymmetric cyanine dyes, which is the same family that SYBR™ Green belongs to. SyGreen is an improved, new generation dye from this family. Similar to SYBR™ Green, SyGreen intercalates the DNA. Unlike SYBR™ Green, SyGreen supports PCR amplification over a wide range of dye concentrations, that is, the dye does not inhibit the PCR reaction. This plays a role in producing robust and consistent DNA melting curves both with prokaryote and eukaryote templates, as it shows no preference in nucleotide composition.
What are the ROX concentrations in qPCRBIO SyGreen Mixes?
The qPCRBIO SyGreen Mixes that contain passive reference dyes come in different formulations, each with a different concentration of the passive reference dye:
- qPCRBIO SyGreen Mix Lo-ROX (PB20.11) contains 112 nM ROX.
- qPCRBIO SyGreen Mix Hi-ROX (PB20.12) contains 1.12 µM ROX.
- qPCRBIO SyGreen Mix Fluorescein (PB20.13) contains 0.2 mM fluorescein.
- qPCRBIO SyGreen Mix Separate-ROX (PB20.14) 2x mix contains no ROX and include a separate tube of 50 µM ROX additive. This enables you to choose what concentration of ROX you’d like to use.
You can use our qPCR Selection Tool under the Resources drop-down menu to determine which of our mixes are best suited for your qPCR machine.
What is included in qPCRBIO SyGreen Mixes?
qPCRBIO SyGreen Mixes are ready to use qPCR 2x Mastermixes. You only need to add primers, template DNA and PCR grade water during reaction set up.
What is ROX?
ROX is a passive reference dye which means it does not take part in the PCR reaction. It is used to normalise non-PCR related fluctuations in fluorescence.
What is the error rate of the HS Taq DNA Polymerase used in qPCRBIO SyGreen Mixes?
The enzyme has an error rate of approximately 1 error per 2.0 x 10⁵ nucleotides incorporated.
What is the MgCl2 concentration in qPCRBIO SyGreen Mixes?
All qPCRBIO SyGreen Mixes contain MgCl2 at a concentration of 6 mM. This means the final concentration in the reaction is 3 mM.
What should be considered if the normalisation of signal with ROX appears to be lower relative to a competitor’s mix?
Ensure you are using the right concentration of ROX because different instruments require different ROX concentrations. For example, if a qPCRBIO SyGreen Mix Hi-ROX is used with an instrument requiring a Lo-ROX mix, the software will normalise the qPCRBIO signal against the Hi-ROX level. This will significantly reduce the fluorescence level of the qPCRBIO mix relative to competitors’ mixes, if in that case the Lo-ROX mix was chosen for the competitor.
When comparing mixes from different manufacturers, it is better to carry out separate runs or turn the passive reference off before analysing data.
What troubleshooting is recommended if efficiency of amplification is reduced with standard dilutions?
It has been reported that efficiency can decrease with subsequent dilutions for the standard curve. We recommend avoiding this by diluting the standards in 10 mM Tris-HCl pH 8.0, 0.1 mM EDTA, 0.05% Tween-20. EDTA is a chelating agent and it plays a role in preventing DNAse activity1. Tween-20 is a detergent and prevents the DNA from adsorbing to the sides of the tubes2. Most microcentrifuges are made of polypropylene and research has demonstrated that DNA sticks very well to polypropylene3.
Standards should not be frozen after diluting them. Even in the presence of detergent, freezing seems to cause DNA to bind irreversibly to polypropylene. We suggest leaving your standards at 4°C and preparing a fresh batch every few weeks.
1 Barra, G. B. et al. EDTA-mediated inhibition of DNases protects circulating cell-free DNA from ex vivo degradation in blood samples. Clin Biochem 48, 976-981, doi:10.1016/j.clinbiochem.2015.02.014 (2015).
2 Linnarsson, S. Recent advances in DNA sequencing methods – general principles of sample preparation. Exp Cell Res 316, 1339-1343, doi:10.1016/j.yexcr.2010.02.036 (2010).
3 Gaillard, C. & Strauss, F. Avoiding adsorption of DNA to polypropylene tubes and denaturation of short DNA fragments. Technical Tips Online 3, 3 (1998).
What troubleshooting is recommended if the background signal is very high?
A high background level of fluorescence is most likely due to an excess of template in the reaction. This relates to the qPCR instrument picking up the SyGreen dye which binds to the template DNA. Samples usually contain a lot of DNA other than the target gene, so there will be enough present to register fluorescence. We recommend diluting the samples 100x-1000x to overcome this issue.
This should not only reduce the background signal, but also allow an accurate quantification because the dilution should bring the Ct values in the range of those obtained with the standards. Keep in mind that if the Ct is earlier than the first standard, it won’t be considered accurate enough quantification for publication.
Why is there a non-specific product when using the same primers and PCR conditions as a competitor’s product?
It’s most likely because the time for the 1st step (hot start) is too short. Ensure that the hot start phase is done at 95°C for 2 minutes to fully activate the enzyme. The recommended thermal profile is:
- 95°C (120 seconds)
- 40 cycles: 95°C (5-15 seconds) – 60°C (20-30 seconds)
- Melt
If non-specific products are still obtained, we recommend raising the annealing/extension temperature from 60°C to 65°C, depending on the primer set used.
Why is there an earlier Ct when using qPCRBIO SyGreen Mixes compared to a competitor’s product?
An early Ct could indicate one of two things. The first could be the result of our product performing very well relative to the competitor’s product. We have designed our products to be faster than our competitors, and we have found that we are significantly faster than many.
The second could be that caused by non-specific products. This can be verified by running a melt analysis at the end of the qPCR run. If your results show one peak, it is likely the correct product was amplified. You may observe the position of the peak being different with our mix relative to our competitor’s mix but is this normal because the salt concentration and pH affect the melting temperature. If your results show more than one peak, then the reaction is not specific. PCR products can also be run on an agarose gel to verify if there is only one band, and if it is the same size as the amplicon obtained with the competitor’s product.
To address the issue of a non-specific product, the thermal cycling will need to be adjusted.