To view your price please login or contact us
Clara™ Probe Mix
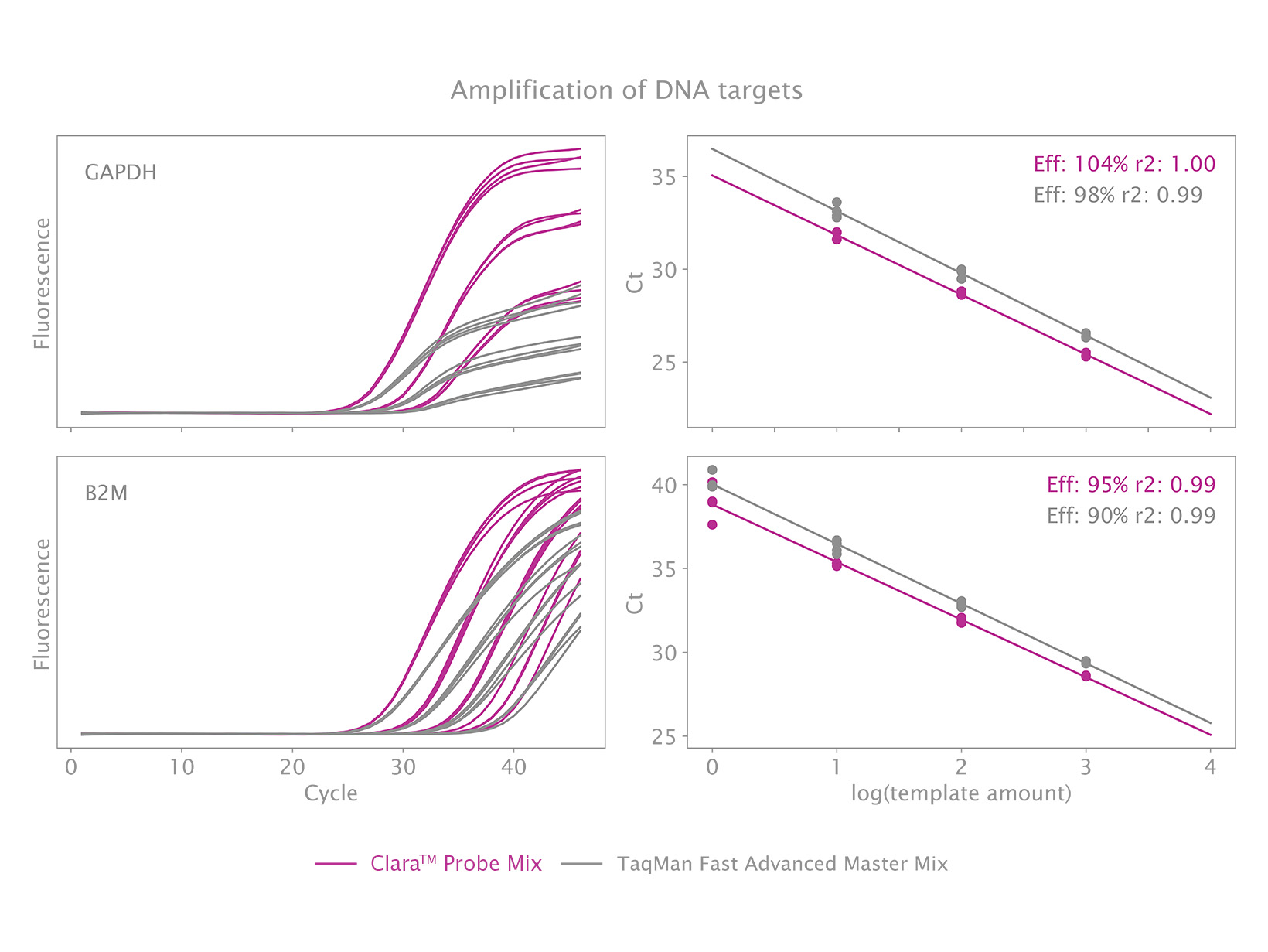
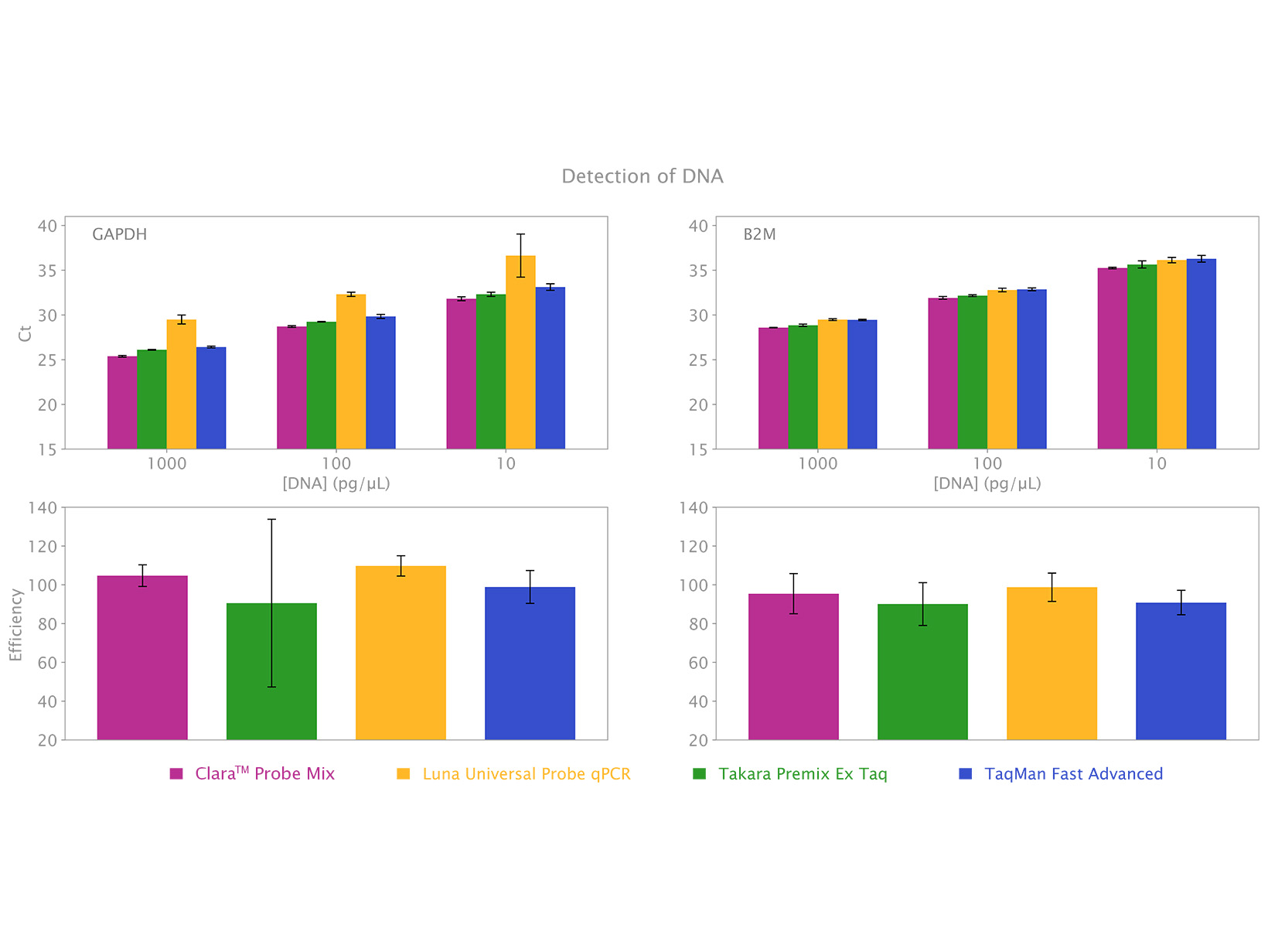
Clara™ Probe Mix is the newest solution for qPCRs. With clear and consistent results, this cutting-edge qPCR mix will streamline your real-time PCR workload no matter what the application.
Clara™ Probe Mix is a next-generation qPCR mastermix designed to give you reliable results with superior sensitivity. This optimised 4x formulation is engineered for maximum reliability in both single and multiplex assays. It is suitable for diagnostic test development and basic research and is universally compatible with all probe types and qPCR instruments.
Features
- Concentrated 4x mix format, ideal for high-throughput, highly multiplexed assays
- Superior detection of DNA targets
- Reliable quantification of low template amounts
- Reduced primer dimer formation for high specificity
- Antibody-mediated hot start technology
- Compatible with all real-time PCR platforms – standard and fast cycling conditions
More Information
Reliable Probe qPCR
Clara™ Probe Mix is engineered to enable qPCRs with the highest sensitivity, reliability, and with the greatest ease of use in diagnostic applications and basic research alike. It is a universal qPCR mix suitable for all types of probe technologies, including TaqMan®, Scorpions® and molecular beacons. Powered by our unique hot start Taq DNA polymerase the qPCR mix is suitable for DNA detection for 2-step RT-qPCR protocols when used with a separate cDNA synthesis kit. Although for routine RNA quantification we recommend 1-step protocols with Clara™ Probe 1-Step Mix for a more streamlined workflow.
Clara™ Probe Mix can be used for all types of probe-based qPCR applications, including gene expression analysis, SNP/allele detection, genotyping and allelic discrimination studies, species abundance quantification, and can offers high efficiency in both single and multiplex assays. Making this mix highly versatile and ideal for in-vitro diagnostic kit development as well as basic research.
The mix is also suitable for melt-curve analysis (with hybridisation probes only) and is available without passive reference dye (No-Rox), or with reverence dye as Lo-ROX, Hi-Rox and separate-ROX formulations. Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
Applications
- 2-step RT-qPCR
- Species abundance quantification
- Genotyping
- Allelic discrimination
- In vitro diagnostic kit development
- Single & multiplex DNA detection
Specifications
Clara™ Probe Mix Lo-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
10,000 Reactions
4x Clara™ Probe Mix Lo-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
1 x 50 mL
Clara™ Probe Mix Hi-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
10,000 Reactions
4x Clara™ Probe Mix Hi-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
1 x 50 mL
Clara™ Probe Mix No-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
10,000 Reactions
4x Clara™ Probe Mix No-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
1 x 50 mL
Clara™ Probe Mix Separate-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
4x Clara™ Probe Mix No-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
50 μΜ ROX Additive
1 x 200 μL
1 x 200 μL
1 x 200 μL
Clara™ Probe Mix Lo-ROX
Component
4x Clara™ Probe Mix Lo-ROX
200 Reactions
1 x 1 mL
600 Reactions
3 x 1 mL
1000 Reactions
5 x 1 mL
10,000 Reactions
1 x 50 mL
Clara™ Probe Mix Hi-ROX
Component
4x Clara™ Probe Mix Hi-ROX
200 Reactions
1 x 1 mL
600 Reactions
3 x 1 mL
1000 Reactions
5 x 1 mL
10,000 Reactions
1 x 50 mL
Clara™ Probe Mix No-ROX
Component
4x Clara™ Probe Mix No-ROX
200 Reactions
1 x 1 mL
600 Reactions
3 x 1 mL
1000 Reactions
5 x 1 mL
10,000 Reactions
1 x 50 mL
Clara™ Probe Mix Separate-ROX
Component
4x Clara™ Probe Mix No-ROX
50 μΜ ROX Additive
200 Reactions
1 x 1 mL
1 x 200 μL
600 Reactions
3 x 1 mL
1 x 200 μL
1000 Reactions
5 x 1 mL
1 x 200 μL
Reaction Volume
Storage
20 μL
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Reaction Volume
20 μL
Storage
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Instrument Compatibility
This product is compatible with all standard and fast cycling qPCR instruments. Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
Documents
Product Flyers
Product Manuals
Material Safety Data Sheets
Certificate of Analysis Finder
FAQs
Beyond what Ct value are results unreliable?
Ct values can vary between template concentrations, reaction optimisation, instruments and laboratories, so care must be taken when selecting a cut-off Ct value. Generally, Ct values over 35-40 would begin to be considered unreliable. However, late Cts could be observed for inefficient reactions using low template copy number. It is always good practice to normalise cut-offs with relative or absolute quantification methods. It is also recommended to run and analyse melt curves or gels of the products to determine products of any late amplifications.
Can products generated with Clara™ and Clara™ Purple mixes be digested, cloned, and sequenced?
Yes, PCR products generated with these mixes have the same characteristics as PCR products generated with wild-type Taq polymerase. They may be sequenced or digested with restriction endonucleases using standard protocols. Products are 3′-d(A)-tailed and may be used for TA cloning or may be blunt-ended or digested with restriction enzymes prior to cloning. For best results, we recommend purifying the PCR products using any standard PCR clean-up kit.
Can ROX have a negative impact on the reaction?
ROX (6-carboxy-X-rhodamine) is used as a passive reference dye in ROX-dependent real-time PCR instruments to normalize for variations of fluorescence levels that can arise mainly due to optical path variations among wells. Normalisation of the fluorescence intensity (Rn) is done in real-time PCR software by dividing the emission intensity of the specific signal by the emission intensity of ROX.
ROX does not take part in the PCR reaction and its fluorescence levels are not proportional to the quantity of DNA in each well, so the addition of this fluorophore to a mix provides a constant fluorescent signal during amplification.
Different types of real-time PCR instruments requiring a passive reference standard have different optimal concentrations of ROX, mainly due to the different optical configurations of each system (i.e. the different type of excitation source and optics used).
The addition of either too little or too much ROX would result in a very noisy signal impacting on the results of the reaction. Therefore, it is extremely important for the user to:
- Determine the correct ROX concentration to optimise real-time PCR results, and
- Check the ROX settings on the software used to set up the reaction
A useful selection tool for the most commonly used systems can be found here.
Do Clara™ Probe Mix and Clara™ Probe 1-Step Mix contain the FAM dye in it?
No. Apart from ROX (if present in the kit), there is no other dye in our mixes. You can therefore use any fluorophore-conjugated probe for your reaction.
Is it normal if the fluorescence of Clara™ Probe Mix and Clara™ Probe 1-Step Mix differs from the one obtained with competitors’ products?
Different products could give a different plateau of fluorescence. However, this has no impact on quantification accuracy and Ct values will not differ among products.
Is the storage of sample DNA in 1x TE (10 mM Tris-HCl / 1 mM EDTA) buffer compatible with subsequent qPCR using Clara™ Probe Mix and Clara™ Probe 1-Step Mix?
Yes, this storage buffer is compatible. The EDTA will chelate some of the magnesium in the mix, but not significantly enough to affect the reaction.
What are the ROX concentrations in Clara™ Probe Mix, Clara™ Probe 1-Step Mix and corresponding purple mixes?
The Clara™ Probe Purple and Clara™ Probe 1-Step Purple Mixes that contain passive reference dyes come in different formulations, each with a different concentration of the passive reference dye:
Lo-ROX mixes (PB20.65 and PB25.85) contain 200 nM ROX.
Hi-ROX mixes (PB20.66 and PB25.86) contain 2 µM ROX.
No-ROX mixes (PB20.67 and PB25.87) do not contain ROX.
Separate-ROX mixes (PB20.68 and PB25.88) include a separate tube of 50 µM ROX additive. This enables you to choose what concentration of ROX you’d like to use.
You can use our qPCR Selection Tool under the Resources drop-down menu to determine which of our mixes are best suited for your qPCR machine.
What is ROX and do I need it?
ROX is a passive reference dye which means it does not take part in the PCR reaction. It is used to normalise non-PCR related fluctuations in fluorescence. You can use our qPCR Selection Tool in the Resources section to determine which of our qPCR mixes are best suited for your qPCR machine.
What should I do if the reaction is inhibited?
If inhibition is observed, the amount of template in the reaction can be decreased. This will increase the Ct value but lower the likelihood of inhibitors interfering with the Taq DNA polymerase activity. If this doesn’t work, try adding 0.4-4 mg/ml of BSA to the reaction1,2. Ensure the cycling conditions in our product manual are adhered to.
1 Kreader, C. A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl Environ Microbiol 62, 1102-1106 (1996).
2 Wilson, I. G. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol 63, 3741-3751 (1997).
What should I do when amplification efficiency reduces with serial dilutions?
It has been reported that efficiency can decrease with subsequent dilutions for the standard curve. We recommend avoiding this by diluting the standards in 10 mM Tris-HCl pH 8.0, 0.1 mM EDTA, 0.05% Tween-20. EDTA is a chelating agent and it plays a role in preventing DNAse activity1. Tween-20 is a detergent and prevents the DNA from adsorbing to the sides of the tubes2. Most microcentrifuges are made of polypropylene and research has demonstrated that DNA sticks very well to polypropylene3.
Standards should not be frozen after diluting them. Even in the presence of detergent, freezing seems to cause DNA to bind irreversibly to polypropylene. We suggest leaving your standards at 4°C and preparing a fresh batch every few weeks.
1 Barra, G. B. et al. EDTA-mediated inhibition of DNases protects circulating cell-free DNA from ex vivo degradation in blood samples. Clin Biochem 48, 976-981, doi:10.1016/j.clinbiochem.2015.02.014 (2015).
2 Linnarsson, S. Recent advances in DNA sequencing methods – general principles of sample preparation. Exp Cell Res 316, 1339-1343, doi:10.1016/j.yexcr.2010.02.036 (2010).
3 Gaillard, C. & Strauss, F. Avoiding adsorption of DNA to polypropylene tubes and denaturation of short DNA fragments. Technical Tips Online 3, 3 (1998).
What troubleshooting is recommended if there are non-specific products in my qPCR?
There are different options to consider when optimising the reaction:
Reduce the annealing/extension time to 5 seconds
Increase the annealing/extension temperature from 60 to 65°C
Dilute the DNA template by starting with 5ng of DNA and using a 10x template dilution series. In addition to running these on a gel to see if the non-specific products persist, the efficiency of the reaction can be calculated with the software of the qPCR instrument after doing the template dilution. If the efficiency is between 90 – 110%, then the amplicon is being doubled every cycle.
What troubleshooting is recommended when Ct values are higher than usual?
Higher Ct values are generally indicative of delayed amplification. This could most likely be due to an excess of template in the reaction resulting in the primers and probes being bound on different DNA molecules. Samples usually have a lot of DNA other than the target gene and this can scatter the oligos. We recommend diluting the samples (10x-1000x) to solve this.
Moreover, annealing/extension temperature can also be increased to make the binding of the oligos more specific to the target sequence and decrease background signal.
When performing a multiplex, what is the recommended concentration of each primer?
We recommend using 0.4 µM of each primer. There is a degree of flexibility around this recommended concentration however, the primer concentration should not be increased, as this may significantly affect the activity of the enzyme.
For further considerations on multiplexing please refer to our qPCR Technical Guide.
Why is there a non-specific product when using the same primers and PCR conditions as a competitor’s product?
It’s most likely because the time for the 1st step (hot start) is too short. Ensure that the hot start phase is done at 95°C for 2 minutes to fully activate the enzyme. The recommended thermal profile is:
- 95°C (120 seconds)
- 40 cycles: 95°C (5-15 seconds) – 60°C (20-30 seconds)
- Melt
If non-specific products are still obtained, we recommend raising the annealing/extension temperature from 60°C to 65°C, depending on the primer set used.
More Information
Reliable Probe qPCR
Clara™ Probe Mix is engineered to enable qPCRs with the highest sensitivity, reliability, and with the greatest ease of use in diagnostic applications and basic research alike. It is a universal qPCR mix suitable for all types of probe technologies, including TaqMan®, Scorpions® and molecular beacons. Powered by our unique hot start Taq DNA polymerase the qPCR mix is suitable for DNA detection for 2-step RT-qPCR protocols when used with a separate cDNA synthesis kit. Although for routine RNA quantification we recommend 1-step protocols with Clara™ Probe 1-Step Mix for a more streamlined workflow.
Clara™ Probe Mix can be used for all types of probe-based qPCR applications, including gene expression analysis, SNP/allele detection, genotyping and allelic discrimination studies, species abundance quantification, and can offers high efficiency in both single and multiplex assays. Making this mix highly versatile and ideal for in-vitro diagnostic kit development as well as basic research.
The mix is also suitable for melt-curve analysis (with hybridisation probes only) and is available without passive reference dye (No-Rox), or with reverence dye as Lo-ROX, Hi-Rox and separate-ROX formulations. Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
Applications
- 2-step RT-qPCR
- Species abundance quantification
- Genotyping
- Allelic discrimination
- In vitro diagnostic kit development
- Single & multiplex DNA detection
Specifications
Clara™ Probe Mix Lo-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
10,000 Reactions
4x Clara™ Probe Mix Lo-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
1 x 50 mL
Clara™ Probe Mix Hi-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
10,000 Reactions
4x Clara™ Probe Mix Hi-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
1 x 50 mL
Clara™ Probe Mix No-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
10,000 Reactions
4x Clara™ Probe Mix No-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
1 x 50 mL
Clara™ Probe Mix Separate-ROX
Component
200 Reactions
600 Reactions
1000 Reactions
4x Clara™ Probe Mix No-ROX
1 x 1 mL
3 x 1 mL
5 x 1 mL
50 μΜ ROX Additive
1 x 200 μL
1 x 200 μL
1 x 200 μL
Clara™ Probe Mix Lo-ROX
Component
4x Clara™ Probe Mix Lo-ROX
200 Reactions
1 x 1 mL
600 Reactions
3 x 1 mL
1000 Reactions
5 x 1 mL
10,000 Reactions
1 x 50 mL
Clara™ Probe Mix Hi-ROX
Component
4x Clara™ Probe Mix Hi-ROX
200 Reactions
1 x 1 mL
600 Reactions
3 x 1 mL
1000 Reactions
5 x 1 mL
10,000 Reactions
1 x 50 mL
Clara™ Probe Mix No-ROX
Component
4x Clara™ Probe Mix No-ROX
200 Reactions
1 x 1 mL
600 Reactions
3 x 1 mL
1000 Reactions
5 x 1 mL
10,000 Reactions
1 x 50 mL
Clara™ Probe Mix Separate-ROX
Component
4x Clara™ Probe Mix No-ROX
50 μΜ ROX Additive
200 Reactions
1 x 1 mL
1 x 200 μL
600 Reactions
3 x 1 mL
1 x 200 μL
1000 Reactions
5 x 1 mL
1 x 200 μL
Reaction Volume
Storage
20 μL
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Reaction Volume
20 μL
Storage
On arrival, products should be stored between -30 and -15°C. If stored correctly the kit will retain full activity for 12 months.
Instrument Compatibility
This product is compatible with all standard and fast cycling qPCR instruments. Use our qPCR Selection Tool to find out which ROX variant is compatible with your instrument.
Documents
Product Flyers
Product Manuals
Material Safety Data Sheets
Certificate of Analysis Finder
FAQs
Beyond what Ct value are results unreliable?
Ct values can vary between template concentrations, reaction optimisation, instruments and laboratories, so care must be taken when selecting a cut-off Ct value. Generally, Ct values over 35-40 would begin to be considered unreliable. However, late Cts could be observed for inefficient reactions using low template copy number. It is always good practice to normalise cut-offs with relative or absolute quantification methods. It is also recommended to run and analyse melt curves or gels of the products to determine products of any late amplifications.
Can products generated with Clara™ and Clara™ Purple mixes be digested, cloned, and sequenced?
Yes, PCR products generated with these mixes have the same characteristics as PCR products generated with wild-type Taq polymerase. They may be sequenced or digested with restriction endonucleases using standard protocols. Products are 3′-d(A)-tailed and may be used for TA cloning or may be blunt-ended or digested with restriction enzymes prior to cloning. For best results, we recommend purifying the PCR products using any standard PCR clean-up kit.
Can ROX have a negative impact on the reaction?
ROX (6-carboxy-X-rhodamine) is used as a passive reference dye in ROX-dependent real-time PCR instruments to normalize for variations of fluorescence levels that can arise mainly due to optical path variations among wells. Normalisation of the fluorescence intensity (Rn) is done in real-time PCR software by dividing the emission intensity of the specific signal by the emission intensity of ROX.
ROX does not take part in the PCR reaction and its fluorescence levels are not proportional to the quantity of DNA in each well, so the addition of this fluorophore to a mix provides a constant fluorescent signal during amplification.
Different types of real-time PCR instruments requiring a passive reference standard have different optimal concentrations of ROX, mainly due to the different optical configurations of each system (i.e. the different type of excitation source and optics used).
The addition of either too little or too much ROX would result in a very noisy signal impacting on the results of the reaction. Therefore, it is extremely important for the user to:
- Determine the correct ROX concentration to optimise real-time PCR results, and
- Check the ROX settings on the software used to set up the reaction
A useful selection tool for the most commonly used systems can be found here.
Do Clara™ Probe Mix and Clara™ Probe 1-Step Mix contain the FAM dye in it?
No. Apart from ROX (if present in the kit), there is no other dye in our mixes. You can therefore use any fluorophore-conjugated probe for your reaction.
Is it normal if the fluorescence of Clara™ Probe Mix and Clara™ Probe 1-Step Mix differs from the one obtained with competitors’ products?
Different products could give a different plateau of fluorescence. However, this has no impact on quantification accuracy and Ct values will not differ among products.
Is the storage of sample DNA in 1x TE (10 mM Tris-HCl / 1 mM EDTA) buffer compatible with subsequent qPCR using Clara™ Probe Mix and Clara™ Probe 1-Step Mix?
Yes, this storage buffer is compatible. The EDTA will chelate some of the magnesium in the mix, but not significantly enough to affect the reaction.
What are the ROX concentrations in Clara™ Probe Mix, Clara™ Probe 1-Step Mix and corresponding purple mixes?
The Clara™ Probe Purple and Clara™ Probe 1-Step Purple Mixes that contain passive reference dyes come in different formulations, each with a different concentration of the passive reference dye:
Lo-ROX mixes (PB20.65 and PB25.85) contain 200 nM ROX.
Hi-ROX mixes (PB20.66 and PB25.86) contain 2 µM ROX.
No-ROX mixes (PB20.67 and PB25.87) do not contain ROX.
Separate-ROX mixes (PB20.68 and PB25.88) include a separate tube of 50 µM ROX additive. This enables you to choose what concentration of ROX you’d like to use.
You can use our qPCR Selection Tool under the Resources drop-down menu to determine which of our mixes are best suited for your qPCR machine.
What is ROX and do I need it?
ROX is a passive reference dye which means it does not take part in the PCR reaction. It is used to normalise non-PCR related fluctuations in fluorescence. You can use our qPCR Selection Tool in the Resources section to determine which of our qPCR mixes are best suited for your qPCR machine.
What should I do if the reaction is inhibited?
If inhibition is observed, the amount of template in the reaction can be decreased. This will increase the Ct value but lower the likelihood of inhibitors interfering with the Taq DNA polymerase activity. If this doesn’t work, try adding 0.4-4 mg/ml of BSA to the reaction1,2. Ensure the cycling conditions in our product manual are adhered to.
1 Kreader, C. A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl Environ Microbiol 62, 1102-1106 (1996).
2 Wilson, I. G. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol 63, 3741-3751 (1997).
What should I do when amplification efficiency reduces with serial dilutions?
It has been reported that efficiency can decrease with subsequent dilutions for the standard curve. We recommend avoiding this by diluting the standards in 10 mM Tris-HCl pH 8.0, 0.1 mM EDTA, 0.05% Tween-20. EDTA is a chelating agent and it plays a role in preventing DNAse activity1. Tween-20 is a detergent and prevents the DNA from adsorbing to the sides of the tubes2. Most microcentrifuges are made of polypropylene and research has demonstrated that DNA sticks very well to polypropylene3.
Standards should not be frozen after diluting them. Even in the presence of detergent, freezing seems to cause DNA to bind irreversibly to polypropylene. We suggest leaving your standards at 4°C and preparing a fresh batch every few weeks.
1 Barra, G. B. et al. EDTA-mediated inhibition of DNases protects circulating cell-free DNA from ex vivo degradation in blood samples. Clin Biochem 48, 976-981, doi:10.1016/j.clinbiochem.2015.02.014 (2015).
2 Linnarsson, S. Recent advances in DNA sequencing methods – general principles of sample preparation. Exp Cell Res 316, 1339-1343, doi:10.1016/j.yexcr.2010.02.036 (2010).
3 Gaillard, C. & Strauss, F. Avoiding adsorption of DNA to polypropylene tubes and denaturation of short DNA fragments. Technical Tips Online 3, 3 (1998).
What troubleshooting is recommended if there are non-specific products in my qPCR?
There are different options to consider when optimising the reaction:
Reduce the annealing/extension time to 5 seconds
Increase the annealing/extension temperature from 60 to 65°C
Dilute the DNA template by starting with 5ng of DNA and using a 10x template dilution series. In addition to running these on a gel to see if the non-specific products persist, the efficiency of the reaction can be calculated with the software of the qPCR instrument after doing the template dilution. If the efficiency is between 90 – 110%, then the amplicon is being doubled every cycle.
What troubleshooting is recommended when Ct values are higher than usual?
Higher Ct values are generally indicative of delayed amplification. This could most likely be due to an excess of template in the reaction resulting in the primers and probes being bound on different DNA molecules. Samples usually have a lot of DNA other than the target gene and this can scatter the oligos. We recommend diluting the samples (10x-1000x) to solve this.
Moreover, annealing/extension temperature can also be increased to make the binding of the oligos more specific to the target sequence and decrease background signal.
When performing a multiplex, what is the recommended concentration of each primer?
We recommend using 0.4 µM of each primer. There is a degree of flexibility around this recommended concentration however, the primer concentration should not be increased, as this may significantly affect the activity of the enzyme.
For further considerations on multiplexing please refer to our qPCR Technical Guide.
Why is there a non-specific product when using the same primers and PCR conditions as a competitor’s product?
It’s most likely because the time for the 1st step (hot start) is too short. Ensure that the hot start phase is done at 95°C for 2 minutes to fully activate the enzyme. The recommended thermal profile is:
- 95°C (120 seconds)
- 40 cycles: 95°C (5-15 seconds) – 60°C (20-30 seconds)
- Melt
If non-specific products are still obtained, we recommend raising the annealing/extension temperature from 60°C to 65°C, depending on the primer set used.