PCRBIO HS Taq DNA Polymerase is an advanced antibody-mediated hot start Taq DNA polymerase designed for fast, highly specific and sensitive PCR.
Whether you need a hot start assay for high-throughput, automated reaction setup or the detection of a low copy number template, PCR Biosystems offers you a robust industry-leading enzyme to meet your needs.
Features
- Hot start technology for unrivalled detection of low copy number templates
- Increased PCR success rates with amplicons up to 6kb
- Ultra-low background DNA
- Advanced buffer chemistry including Mg and dNTP
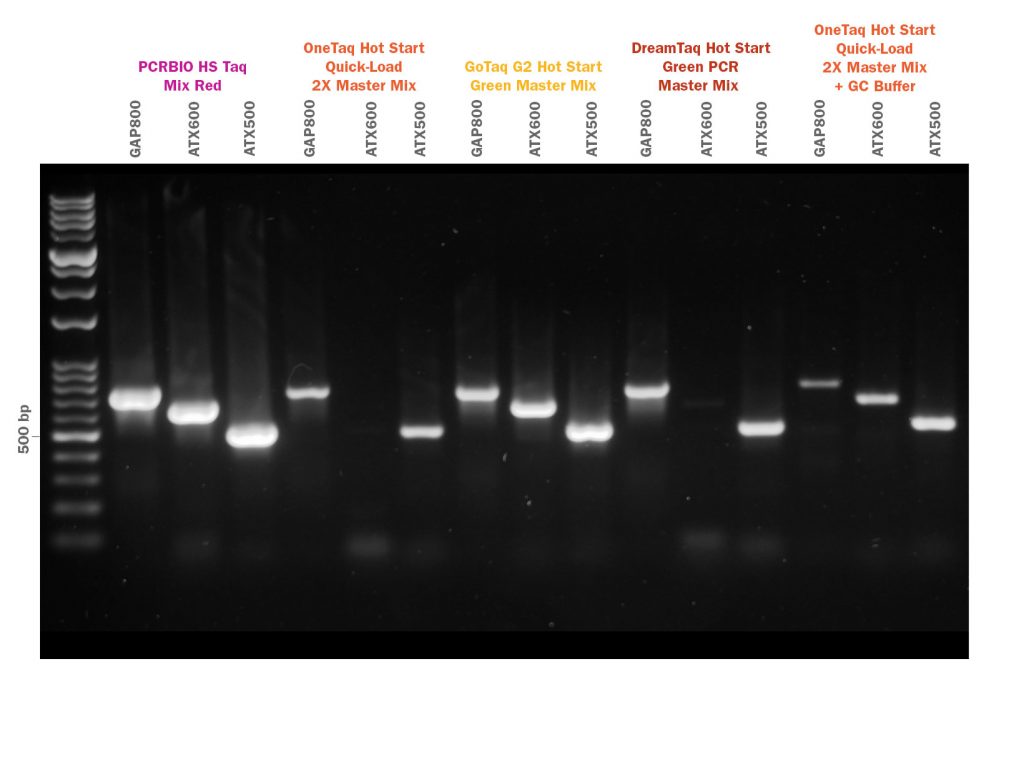
- Efficient specific amplification from GC and AT-rich sequences
- High performance under both fast and standard cycling conditions
- Gives increased PCR throughput with existing thermal cyclers
Applications
- Genotyping
- High-throughput PCR
- Standard and fast PCR
- Routine and multiplex PCR
- TA cloning
- Colony PCR
- ‘Difficult’ PCR – GC and AT-rich DNA
Overview
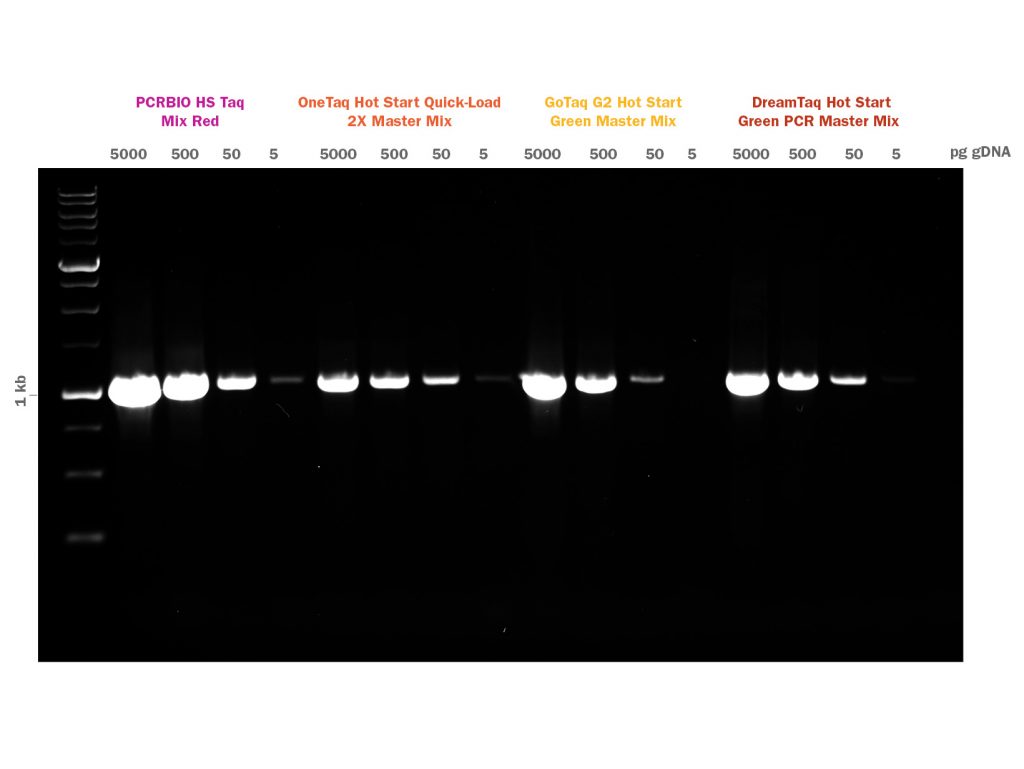
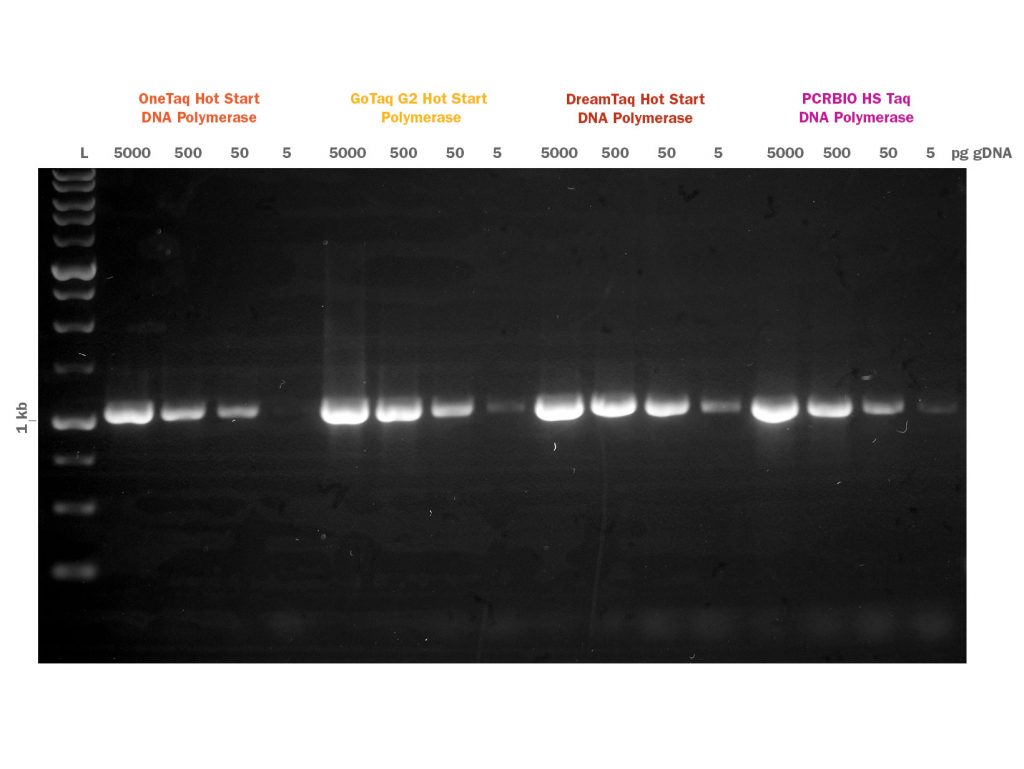
PCRBIO HS Taq DNA Polymerase is an advanced antibody-mediated hot start enzyme engineered for fast, highly specific and sensitive PCR. The latest developments in polymerase technology and buffer chemistry enhance PCR speed, yield and specificity, giving a consistently high performance on a broad range of templates including both GC and AT-rich sequences.
Proprietary antibodies inhibit polymerase activity until an initial activation step at 95°C, preventing the formation of primer dimers and non-specific products, giving improved specificity and sensitivity compared to other methods. The enzyme and buffer system allow for superior PCR performance on complex templates such as mammalian genomic DNA. Whether you need a hot start assay for high-throughput, automated reaction setup or the detection of a low copy number template, PCR Biosystems offers you a robust industry-leading enzyme to meet your needs.
PCRBIO HS Taq DNA Polymerase is also available as a convenient, ready-to-use 2x mix containing all reaction components except primers and template. PCRBIO HS Taq Mix Red contains a preloaded red dye for direct gel loading.
Documents
Product Manuals
Material Safety Data Sheets
Certificate of Analysis Finder
Specifications
PCRBIO HS Taq DNA Polymerase
Component
250 Units
1000 Units
5000 Units
PCRBIO HS Taq DNA Polymerase (5u/μL)
1 x 50 μL
4 x 50 μL
20 x 50 μL
5x PCRBIO Reaction Buffer
2 x 1 mL
8 x 1 mL
40 x 1 mL
PCRBIO HS Taq Mix
Component
200 Reactions
1000 Reactions
2x PCRBIO HS Taq Mix
5 x 1 mL
25 x 1 mL
PCRBIO HS Taq Mix Red
Component
200 Reactions
1000 Reactions
2x PCRBIO HS Taq Mix Red
5 x 1 mL
25 x 1 mL
HS Taq DNA Polymerase (250 U/μL)
Component
5000 Units
Taq DNA Polymerase (250 U/μL)
1 x 20 μL
HS Taq Antibody (10 mg/mL)
1 x 67 μL
HS Taq Antibody (10 mg/mL)
Component
5000 units
HS Taq Antibody (10 mg/mL)
1 x 67 μL
PCRBIO HS Taq DNA Polymerase
Component
PCRBIO HS Taq DNA Polymerase (5u/μL)
5x PCRBIO Reaction Buffer
250 Units
1 x 50 μL
2 x 1 mL
1000 Units
4 x 50 μL
8 x 1 mL
5000 Units
20 x 50 μL
40 x 1 mL
PCRBIO HS Taq Mix
Component
2x PCRBIO HS Taq Mix
200 Reactions
5 x 1 mL
1000 Reactions
25 x 1 mL
PCRBIO HS Taq Mix Red
Component
2x PCRBIO HS Taq Mix Red
200 Reactions
5 x 1 mL
1000 Reactions
25 x 1 mL
HS Taq DNA Polymerase (250 U/μL)
Component
Taq DNA Polymerase (250 U/μL)
HS Taq Antibody (10 mg/mL)
5000 Units
1 x 20 μL
1 x 67 μL
HS Taq Antibody (10 mg/mL)
Component
HS Taq Antibody (10 mg/mL)
5000 units
1 x 67 μL
Reaction Volume
Storage
50μL
On arrival, products should be stored between -30 and -20 °C. If stored correctly the kit will retain full activity until the indicated expiry date.
Reaction Volume
50μL
Storage
On arrival, products should be stored between -30 and -20 °C. If stored correctly the kit will retain full activity until the indicated expiry date.
FAQs
What is the apparent Mw of the Red Mix dye on agarose gels?
This non-inhibitory dye, added to enable direct gel loading, runs at a similar rate to 200-300 bp DNA fragments on a 1% agarose gel and at 50-100 bp on a 2% agarose gel. You may notice a shift in this apparent molecular weight when running gels of different agarose content.
Does PCRBIO HS Taq DNA Polymerase generate an A-tail or blunt end on my PCR product?
PCR products generated with PCRBIO HS Taq DNA Polymerase are A-tailed and this makes it suitable for cloning into TA vectors. For further reading, refer to this literature1.
1 Yao, S., Hart, D. J. & An, Y. Recent advances in universal TA cloning methods for use in function studies. Protein Eng Des Sel, doi:10.1093/protein/gzw047 (2016).
Can PCRBIO HS Taq DNA Polymerase be used for colony PCR?
Yes. If you’re working from bacterial colonies use a sterile tip to pick a colony and re-suspend into the 50µl PCR reaction. If working from liquid culture add 5µl of overnight culture to the final mix. Follow the general protocol and increase the initial denaturation time to 10 min at 95°C.
Can PCRBIO HS Taq DNA Polymerase be used to amplify DNA from blood samples?
Yes. Use 2 µL blood sample to a 50 µL PCR reaction and follow the general protocol. Please note that blood components may inhibit the PCR reaction. Perform a serial dilution of the sample in order to find the optimal template concentration for the PCR amplification.
Can PCRBIO Hot Start Taq DNA Polymerase be used for Multiplex PCR?
Our PCRBIO Hot Start Taq DNA Polymerase is particularly suited for multiplexing due to the presence of our hot-start technology, which blocks the activity of Taq polymerase at low temperatures.
When first performing multiplex PCR, we recommend running an annealing temperature gradient from 55°C to 65°C. The annealing temperature that results in the best specificity should be used in subsequent experiments. Fast cycling conditions should not be used for multiplex PCR. We recommend a 90 second extension time to begin with and this time may be extended to increase yield.
For multiplex reactions, the reactions should be set up on ice or cooling blocs from start till finish. Primers must be designed carefully to avoid overlapping sequences as much as possible while maintaining diverse amplicon lengths that can be easily analysed with your end-detection method1-3.
1 Markoulatos, P., Siafakas, N. & Moncany, M. Multiplex polymerase chain reaction: a practical approach. J Clin Lab Anal 16, 47-51, doi:10.1002/jcla.2058 (2002).
2 Radhika, M., Saugata, M., Murali, H. S. & Batra, H. V. A novel multiplex PCR for the simultaneous detection of Salmonella enterica and Shigella species. Brazilian Journal of Microbiology 45, 667-676, doi:10.1590/s1517-83822014005000041 (2014).
3 Perez-Perez, F. J. & Hanson, N. D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40, 2153-2162, doi:10.1128/jcm.40.6.2153-2162.2002 (2002).
Can PCRBIO HS Taq DNA Polymerase proofread?
No. PCRBIO HS Taq DNA Polymerase has 5’-3’ exonuclease activities, but no 3’-5’ exonuclease (proofreading) activity.
What is the error rate of PCRBIO Hot Start Taq DNA Polymerase?
The enzyme has an error rate of approximately 1 error per 2.0 x 10⁵ nucleotides incorporated.
What is the difference between PCRBIO Taq DNA Polymerase and PCRBIO HS Taq DNA Polymerase?
PCRBIO HS Taq DNA Polymerase uses proprietary antibody-mediated hot start technology whereas PCRBIO Taq DNA Polymerase does not have this feature. The interaction of the antibody with the Taq DNA Polymerase leaves the enzyme inactive until the hot start step.
The hot start refers to the initial activation step at 95°C, which subsequently deactivates the antibody bound to the DNA polymerase. Inactivation of the Taq DNA Polymerase below 65°C prevents primer-dimer formation and non-specific amplification. This allows for specific amplification from low copy number target sequences.
What is the recommended extension time for PCRBIO Hot Start Taq DNA Polymerase?
For amplicons between 1kb and 5kb, we recommend 15 seconds/kb for amplification from eukaryotic DNA. For shorter amplicons, a 1 second extension is sufficient.
My results contain a high background of non-specific amplicons or smears. What trouble-shooting suggestions do you have?
If smears are a concern, it’s good practice to ensure they are not an artefact of running agarose gel electrophoresis with suboptimal conditions. Suboptimal conditions can include high voltage or not allowing enough time for the gel to set1.
You may also need to troubleshoot the PCR reaction and consider the suggestions below2.
- Primers should be designed to prevent primer-primer interactions and improve specificity.
- Increase the annealing temperature or conducting an annealing temperature gradient PCR to determine the optimal annealing temperature.
- Reduce the amount of template in the reaction. For high quality DNA, use 1–100 ng genomic DNA or ≤5 ng plasmid/lambda DNA per 50 µL reaction.
- Reduce the number of cycles.
- Reduce the amount of enzyme per reaction.
- Reduce the primer concentration, but not lower than 100 nM of each primer.
- Include DMSO in the reaction to a final concentration of 5%–10%.
1 Koontz, L. Agarose Gel Electrophoresis. Laboratory methods in enzymology : DNA. First edition. edn, Vol. 529 35-45 (2013).
2 Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp, e3998, doi:10.3791/3998 (2012).
My results show a very low yield. What trouble-shooting suggestions do you have?
You may want to consider the suggestions below and also refer to the literature1.
- Optimise the annealing temperature in an annealing temperature gradient PCR.
- Increase the amount of template in the reaction.
- Increase the number of cycles.
- Increase the amount of enzyme per reaction.
- Increase the primer concentration, but do not exceed 1 µM of each primer.
- Try a fresh dNTP solution.
- Optimise the MgCl2
1 Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp, e3998, doi:10.3791/3998 (2012).
Features
- Hot start technology for unrivalled detection of low copy number templates
- Increased PCR success rates with amplicons up to 6kb
- Ultra-low background DNA
- Advanced buffer chemistry including Mg and dNTP
- Efficient specific amplification from GC and AT-rich sequences
- High performance under both fast and standard cycling conditions
- Gives increased PCR throughput with existing thermal cyclers
Applications
- Genotyping
- High-throughput PCR
- Standard and fast PCR
- Routine and multiplex PCR
- TA cloning
- Colony PCR
- ‘Difficult’ PCR – GC and AT-rich DNA
Overview
PCRBIO HS Taq DNA Polymerase is an advanced antibody-mediated hot start enzyme engineered for fast, highly specific and sensitive PCR. The latest developments in polymerase technology and buffer chemistry enhance PCR speed, yield and specificity, giving a consistently high performance on a broad range of templates including both GC and AT-rich sequences.
Proprietary antibodies inhibit polymerase activity until an initial activation step at 95°C, preventing the formation of primer dimers and non-specific products, giving improved specificity and sensitivity compared to other methods. The enzyme and buffer system allow for superior PCR performance on complex templates such as mammalian genomic DNA. Whether you need a hot start assay for high-throughput, automated reaction setup or the detection of a low copy number template, PCR Biosystems offers you a robust industry-leading enzyme to meet your needs.
PCRBIO HS Taq DNA Polymerase is also available as a convenient, ready-to-use 2x mix containing all reaction components except primers and template. PCRBIO HS Taq Mix Red contains a preloaded red dye for direct gel loading.



Request a Quote & Sample
Your Selection:
Documents
Product Manuals
Material Safety Data Sheets
Certificate of Analysis Finder
Specifications
PCRBIO HS Taq DNA Polymerase
Component
250 Units
1000 Units
5000 Units
PCRBIO HS Taq DNA Polymerase (5u/μL)
1 x 50 μL
4 x 50 μL
20 x 50 μL
5x PCRBIO Reaction Buffer
2 x 1 mL
8 x 1 mL
40 x 1 mL
PCRBIO HS Taq Mix
Component
200 Reactions
1000 Reactions
2x PCRBIO HS Taq Mix
5 x 1 mL
25 x 1 mL
PCRBIO HS Taq Mix Red
Component
200 Reactions
1000 Reactions
2x PCRBIO HS Taq Mix Red
5 x 1 mL
25 x 1 mL
HS Taq DNA Polymerase (250 U/μL)
Component
5000 Units
Taq DNA Polymerase (250 U/μL)
1 x 20 μL
HS Taq Antibody (10 mg/mL)
1 x 67 μL
HS Taq Antibody (10 mg/mL)
Component
5000 units
HS Taq Antibody (10 mg/mL)
1 x 67 μL
PCRBIO HS Taq DNA Polymerase
Component
PCRBIO HS Taq DNA Polymerase (5u/μL)
5x PCRBIO Reaction Buffer
250 Units
1 x 50 μL
2 x 1 mL
1000 Units
4 x 50 μL
8 x 1 mL
5000 Units
20 x 50 μL
40 x 1 mL
PCRBIO HS Taq Mix
Component
2x PCRBIO HS Taq Mix
200 Reactions
5 x 1 mL
1000 Reactions
25 x 1 mL
PCRBIO HS Taq Mix Red
Component
2x PCRBIO HS Taq Mix Red
200 Reactions
5 x 1 mL
1000 Reactions
25 x 1 mL
HS Taq DNA Polymerase (250 U/μL)
Component
Taq DNA Polymerase (250 U/μL)
HS Taq Antibody (10 mg/mL)
5000 Units
1 x 20 μL
1 x 67 μL
HS Taq Antibody (10 mg/mL)
Component
HS Taq Antibody (10 mg/mL)
5000 units
1 x 67 μL
Reaction Volume
Storage
50μL
On arrival, products should be stored between -30 and -20 °C. If stored correctly the kit will retain full activity until the indicated expiry date.
Reaction Volume
50μL
Storage
On arrival, products should be stored between -30 and -20 °C. If stored correctly the kit will retain full activity until the indicated expiry date.
FAQs
What is the apparent Mw of the Red Mix dye on agarose gels?
This non-inhibitory dye, added to enable direct gel loading, runs at a similar rate to 200-300 bp DNA fragments on a 1% agarose gel and at 50-100 bp on a 2% agarose gel. You may notice a shift in this apparent molecular weight when running gels of different agarose content.
Does PCRBIO HS Taq DNA Polymerase generate an A-tail or blunt end on my PCR product?
PCR products generated with PCRBIO HS Taq DNA Polymerase are A-tailed and this makes it suitable for cloning into TA vectors. For further reading, refer to this literature1.
1 Yao, S., Hart, D. J. & An, Y. Recent advances in universal TA cloning methods for use in function studies. Protein Eng Des Sel, doi:10.1093/protein/gzw047 (2016).
Can PCRBIO HS Taq DNA Polymerase be used for colony PCR?
Yes. If you’re working from bacterial colonies use a sterile tip to pick a colony and re-suspend into the 50µl PCR reaction. If working from liquid culture add 5µl of overnight culture to the final mix. Follow the general protocol and increase the initial denaturation time to 10 min at 95°C.
Can PCRBIO HS Taq DNA Polymerase be used to amplify DNA from blood samples?
Yes. Use 2 µL blood sample to a 50 µL PCR reaction and follow the general protocol. Please note that blood components may inhibit the PCR reaction. Perform a serial dilution of the sample in order to find the optimal template concentration for the PCR amplification.
Can PCRBIO Hot Start Taq DNA Polymerase be used for Multiplex PCR?
Our PCRBIO Hot Start Taq DNA Polymerase is particularly suited for multiplexing due to the presence of our hot-start technology, which blocks the activity of Taq polymerase at low temperatures.
When first performing multiplex PCR, we recommend running an annealing temperature gradient from 55°C to 65°C. The annealing temperature that results in the best specificity should be used in subsequent experiments. Fast cycling conditions should not be used for multiplex PCR. We recommend a 90 second extension time to begin with and this time may be extended to increase yield.
For multiplex reactions, the reactions should be set up on ice or cooling blocs from start till finish. Primers must be designed carefully to avoid overlapping sequences as much as possible while maintaining diverse amplicon lengths that can be easily analysed with your end-detection method1-3.
1 Markoulatos, P., Siafakas, N. & Moncany, M. Multiplex polymerase chain reaction: a practical approach. J Clin Lab Anal 16, 47-51, doi:10.1002/jcla.2058 (2002).
2 Radhika, M., Saugata, M., Murali, H. S. & Batra, H. V. A novel multiplex PCR for the simultaneous detection of Salmonella enterica and Shigella species. Brazilian Journal of Microbiology 45, 667-676, doi:10.1590/s1517-83822014005000041 (2014).
3 Perez-Perez, F. J. & Hanson, N. D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40, 2153-2162, doi:10.1128/jcm.40.6.2153-2162.2002 (2002).
Can PCRBIO HS Taq DNA Polymerase proofread?
No. PCRBIO HS Taq DNA Polymerase has 5’-3’ exonuclease activities, but no 3’-5’ exonuclease (proofreading) activity.
What is the error rate of PCRBIO Hot Start Taq DNA Polymerase?
The enzyme has an error rate of approximately 1 error per 2.0 x 10⁵ nucleotides incorporated.
What is the difference between PCRBIO Taq DNA Polymerase and PCRBIO HS Taq DNA Polymerase?
PCRBIO HS Taq DNA Polymerase uses proprietary antibody-mediated hot start technology whereas PCRBIO Taq DNA Polymerase does not have this feature. The interaction of the antibody with the Taq DNA Polymerase leaves the enzyme inactive until the hot start step.
The hot start refers to the initial activation step at 95°C, which subsequently deactivates the antibody bound to the DNA polymerase. Inactivation of the Taq DNA Polymerase below 65°C prevents primer-dimer formation and non-specific amplification. This allows for specific amplification from low copy number target sequences.
What is the recommended extension time for PCRBIO Hot Start Taq DNA Polymerase?
For amplicons between 1kb and 5kb, we recommend 15 seconds/kb for amplification from eukaryotic DNA. For shorter amplicons, a 1 second extension is sufficient.
My results contain a high background of non-specific amplicons or smears. What trouble-shooting suggestions do you have?
If smears are a concern, it’s good practice to ensure they are not an artefact of running agarose gel electrophoresis with suboptimal conditions. Suboptimal conditions can include high voltage or not allowing enough time for the gel to set1.
You may also need to troubleshoot the PCR reaction and consider the suggestions below2.
- Primers should be designed to prevent primer-primer interactions and improve specificity.
- Increase the annealing temperature or conducting an annealing temperature gradient PCR to determine the optimal annealing temperature.
- Reduce the amount of template in the reaction. For high quality DNA, use 1–100 ng genomic DNA or ≤5 ng plasmid/lambda DNA per 50 µL reaction.
- Reduce the number of cycles.
- Reduce the amount of enzyme per reaction.
- Reduce the primer concentration, but not lower than 100 nM of each primer.
- Include DMSO in the reaction to a final concentration of 5%–10%.
1 Koontz, L. Agarose Gel Electrophoresis. Laboratory methods in enzymology : DNA. First edition. edn, Vol. 529 35-45 (2013).
2 Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp, e3998, doi:10.3791/3998 (2012).
My results show a very low yield. What trouble-shooting suggestions do you have?
You may want to consider the suggestions below and also refer to the literature1.
- Optimise the annealing temperature in an annealing temperature gradient PCR.
- Increase the amount of template in the reaction.
- Increase the number of cycles.
- Increase the amount of enzyme per reaction.
- Increase the primer concentration, but do not exceed 1 µM of each primer.
- Try a fresh dNTP solution.
- Optimise the MgCl2
1 Lorenz, T. C. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp, e3998, doi:10.3791/3998 (2012).